training for trust - CET l QAI
CAVF l QAI
Improvement QAI
— CAHSC | QAI.
About Us
Quality and Accreditation Institute (QAI) is a private limited company incorporated by Registrar of Companies under the Companies Act 1956 in India. QAI is an International Accreditation Body that provides accreditation/ certification, education, training and quality improvement activities to various types of organisations including healthcare facilities.
QAI provides a platform to stakeholders including professionals and organisations, associated with quality in any way, to share their wisdom and knowledge. QAI is closely working with stakeholders including government agencies to support accredited facilities in terms of empanelment and other benefits.
Different activities were initiated under different verticals (centres) in a manner that they remain independent of each other. QAI aims to operate globally. Currently, QAI is operating following verticals (centres):
Vision:
Nurturing the largest global pool of organisations and people through quality and accreditation framework.
Mission:
To conceive and deliver education, training, accreditation and related programmes in partnership with stakeholders using an approach of co-design and co-creation.
Our Core Values:
Listener: Seek continuous feedback from stakeholders to address their concerns
Competitive: Look for viable options to benefit users of our services
Transparency: Clearly defined policies made available in public domain
Innovation: Continuously evolve using co-design and co-creation
What We Bring to You
CEO Message

Dr. B.K. Rana, Founding CEO
Quality and Accreditation Institute (QAI)
At the Quality and Accreditation Institute (QAI), our mission is clear: to strengthen trust in accreditation / external evaluation system for various types of organisations and activities through credible, transparent, and globally benchmarked accreditation standards.
Since its inception, QAI has been driven by the belief that accreditation is more than a certificate—it is a culture of accountability, compassion, and continual improvement. We work hand in hand with our stakeholders to embed standards that elevate outcomes, empower our partner organisations and professionals, and inspire public confidence.
As the Founding CEO, I am proud to see QAI emerge as a trusted partner in advancing quality across India and beyond. Our frameworks are aligned with international best practices, yet tailored to local realities, ensuring that organisations of all sizes can embrace excellence.
Looking ahead, QAI will continue to innovate—integrating digital tools, sustainability aspects and performance evaluation into our programmes. Together with our stakeholders, we aim to build a safer, equitable, and more resilient quality ecosystem that serves both present and future generations.
I invite you to join us in this journey of transformation. Accreditation is not just about compliance—it is about commitment. Commitment to beneficiaries, to communities, and to the values that define quality.
Why QAI?
When you choose QAI, you choose credibility, consistency, and confidence in your service delivery. That’s why hundreds of institutions trust QAI to strengthen quality, ensure compliance, and demonstrate commitment to excellence.


QAI Gallery
Discover a captivating gallery that highlights our journey of excellence and the benchmarks we’ve set. Immerse yourself in visual stories of quality, innovation, and achievement.
What Our Customers Say?

KITES Senior Care (A Unit of Lifebridge Senior Care Pvt. Ltd.)

Narayana Nethralaya Institute of Molecular Diagnostics & Laboratory Services, Bengaluru

Max@Home, Gurugram

Guardian Angel Home Care, Ernakulum
Featured News and Insights
Read the latest news about medicross as well as medical news around the world.
FAQs
The trusted partner of hundreds of Organizations in adopting excellence.

1. What is QAI Accreditation?
2. Who can get accreditation?
the applicable quality and safety standards and fulfills
the prescribed assessment requirements.
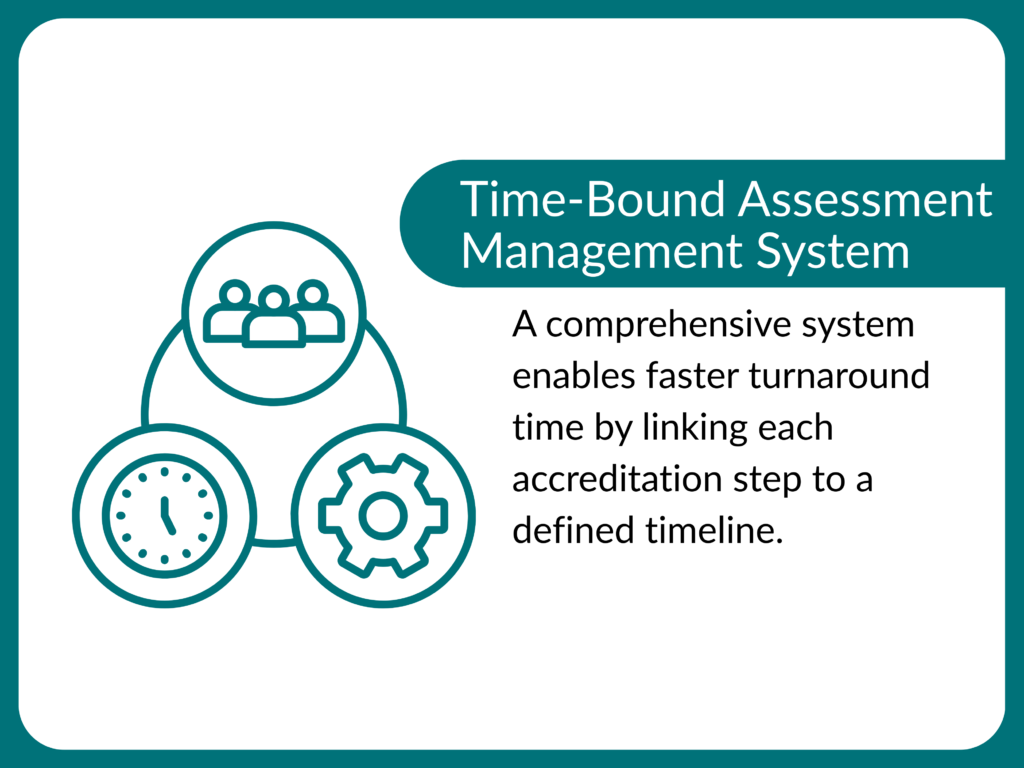
3. What is the turn around time (TAT) of accreditation?
bound, with the complete process typically concluded
in around 60-120 days, provided the applicant
submits complete documents and closes observations
promptly. This structured timeline ensures a
transparent, fair, and credible accreditation outcome
without unnecessary delays.
4.What are the advantages of getting accredited?
5. How to start accreditation process?
relevant accreditation program based on the standard
and go through the information brochure of the
programme. Or you may directly contact QAI via
email at info@qai.org or by phone at +91-
8287841146.